
Figure 1.2 from Part A: Palladium(II)-catalyzed enantioselective Saucy-Marbet Claisen rearrangement of propargyloxy indoles to quaternary oxindoles and spirocyclic lactones. Part B: The regioselective oxidative coupling of phenols | Semantic Scholar

Diastereocontrol via the phenol- and palladium(II)-catalyzed Claisen rearrangement with cyclic enol ethers - ScienceDirect
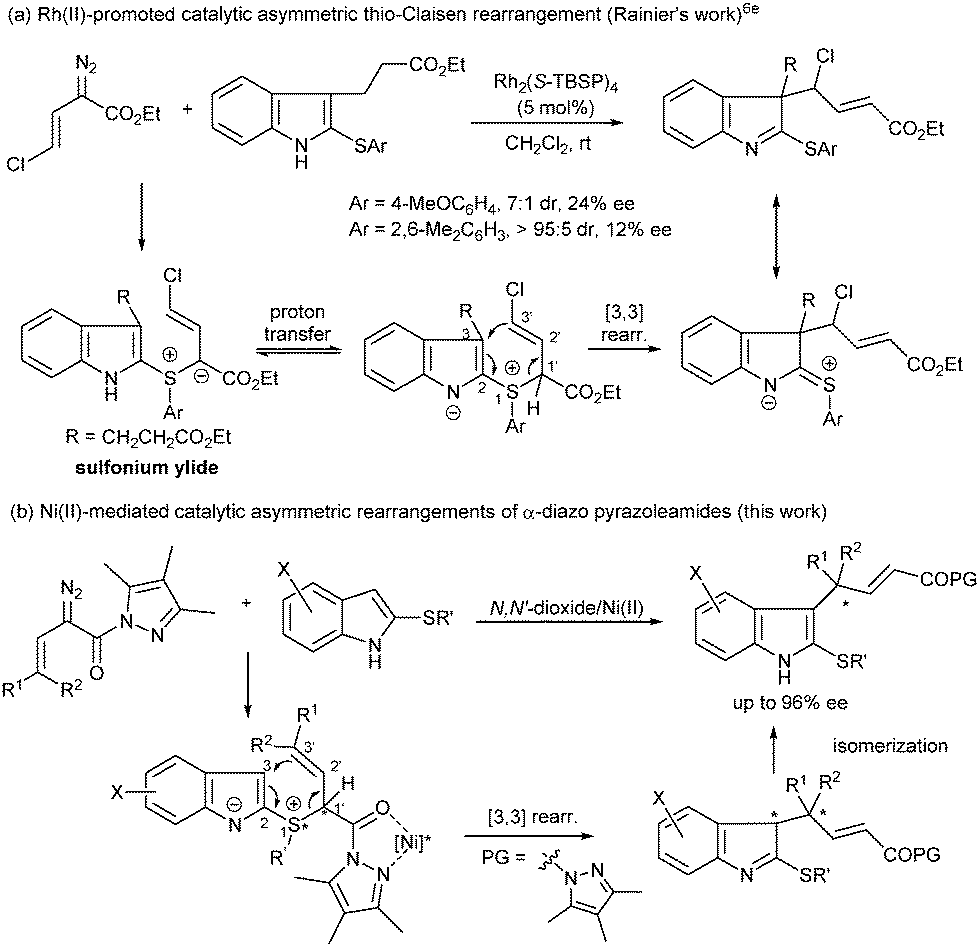
Nickel(ii)-catalyzed asymmetric thio-Claisen rearrangement of α-diazo pyrazoleamides with thioindoles - Chemical Communications (RSC Publishing)

Table 1.4 from Part A: Palladium(II)-catalyzed enantioselective Saucy-Marbet Claisen rearrangement of propargyloxy indoles to quaternary oxindoles and spirocyclic lactones. Part B: The regioselective oxidative coupling of phenols | Semantic Scholar
![PDF) A new palladium(II)-catalyzed [3,3] aza-Claisen rearrangement of 3-allyloxy-5-aryl-1,2,4-oxadiazoles | Antonio Piccionello - Academia.edu PDF) A new palladium(II)-catalyzed [3,3] aza-Claisen rearrangement of 3-allyloxy-5-aryl-1,2,4-oxadiazoles | Antonio Piccionello - Academia.edu](https://0.academia-photos.com/attachment_thumbnails/44982140/mini_magick20190213-14539-zxuyqy.png?1550072610)
PDF) A new palladium(II)-catalyzed [3,3] aza-Claisen rearrangement of 3-allyloxy-5-aryl-1,2,4-oxadiazoles | Antonio Piccionello - Academia.edu

Asymmetric Synthesis of Allenyl Oxindoles and Spirooxindoles by a Catalytic Enantioselective Saucy–Marbet Claisen Rearrangement - Cao - 2012 - Angewandte Chemie International Edition - Wiley Online Library

Toward a symphony of reactivity: cascades involving catalysis and sigmatropic rearrangements. - Abstract - Europe PMC
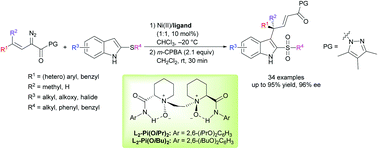
Nickel(ii)-catalyzed asymmetric thio-Claisen rearrangement of α-diazo pyrazoleamides with thioindoles - Chemical Communications (RSC Publishing)
A continuous-flow resonator-type microwave reactor for high-efficiency organic synthesis and Claisen rearrangement as a model re

Top PDF Concerning the mechanism and selectivity of palladium(II)-catalyzed aerobic oxidation reactions - 1Library

Table 2.5 from Part A: Palladium(II)-Catalyzed Enantioselective Saucy-Marbet Claisen Rearrangement of Propargyloxy indoles to Quaternary Oxindoles and Spirocyclic Lactones. Part B: The Regioselective Oxidative Coupling of Phenols . | Semantic Scholar

Table 2.2 from Part A: Palladium(II)-Catalyzed Enantioselective Saucy-Marbet Claisen Rearrangement of Propargyloxy indoles to Quaternary Oxindoles and Spirocyclic Lactones. Part B: The Regioselective Oxidative Coupling of Phenols . | Semantic Scholar

Copper(II)- and Palladium(II)-Catalyzed Enantioselective Claisen Rearrangement of Allyloxy- and Propargyloxy-Indoles





![Palladium catalyzed polyhetero-Claisen rearrangement - [PDF Document] Palladium catalyzed polyhetero-Claisen rearrangement - [PDF Document]](https://reader011.staticloud.net/reader011/html5/20190224/5750a2be1a28abcf0c9d7651/bg1.png)

