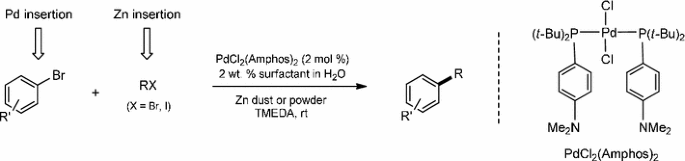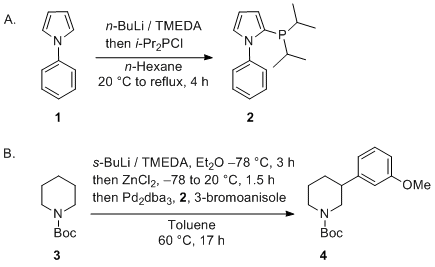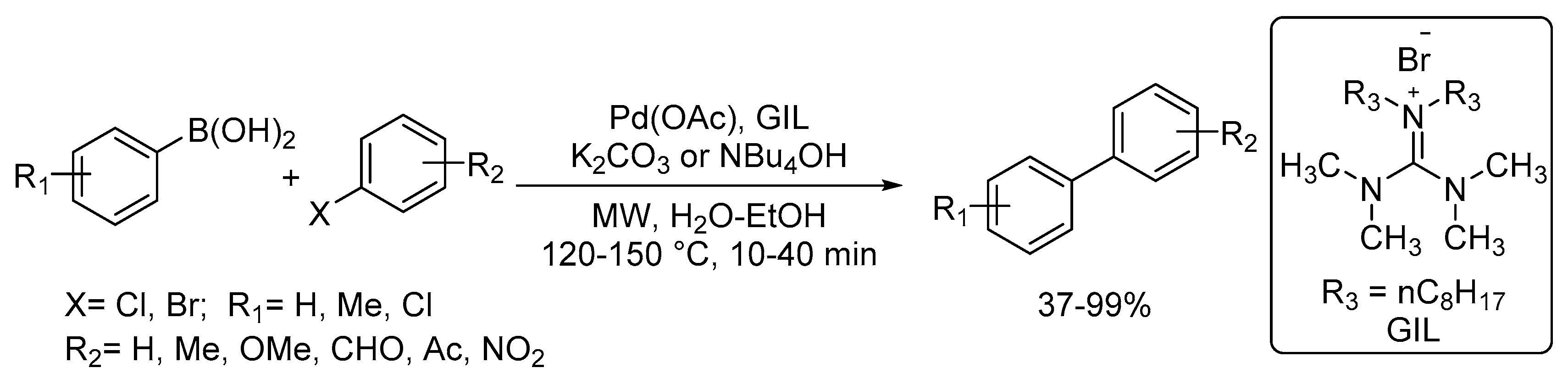
Catalysts | Free Full-Text | Microwave-Assisted Palladium-Catalyzed Cross-Coupling Reactions: Generation of Carbon–Carbon Bond | HTML

Hydrodehalogenation of halogenated pyridines and quinolines by sodium borohydride/N,N,N′,N′-tetramethylethylenediamine under palladium catalysis - ScienceDirect

Synthesis of Internal Alkynes by Pd(PPh3)4/TMEDA‐Catalyzed Kumada Cross‐Coupling of Alkynyl Halides with Grignard Reagents - Zhang - 2014 - European Journal of Organic Chemistry - Wiley Online Library
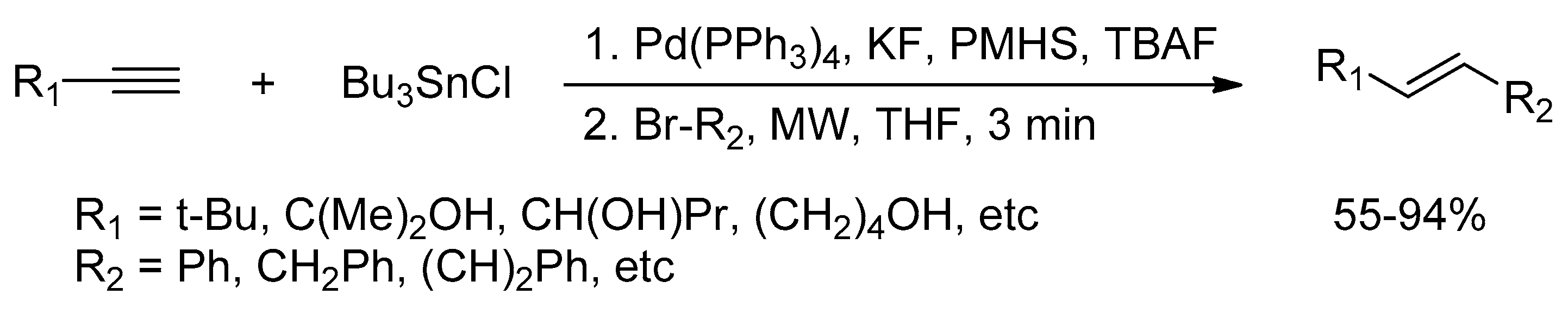
Catalysts | Free Full-Text | Microwave-Assisted Palladium-Catalyzed Cross-Coupling Reactions: Generation of Carbon–Carbon Bond | HTML

Palladium-catalyzed decarboxylative gem-selective addition of alkynoic acids to terminal alkynes - Organic Chemistry Frontiers (RSC Publishing)
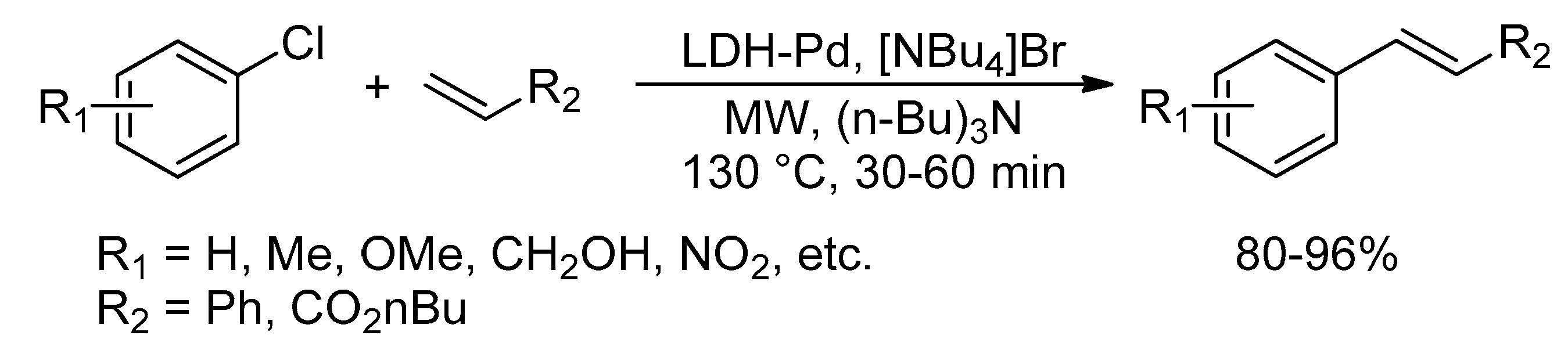
Catalysts | Free Full-Text | Microwave-Assisted Palladium-Catalyzed Cross-Coupling Reactions: Generation of Carbon–Carbon Bond | HTML

Figure 1 from Palladium-catalyzed enantioselective alpha-arylation and alpha-vinylation of oxindoles facilitated by an axially chiral P-stereogenic ligand. | Semantic Scholar

Hydrodehalogenation of halogenated pyridines and quinolines by sodium borohydride/N,N,N′,N′-tetramethylethylenediamine under palladium catalysis

Regioselective and Stereospecific Cross‐Coupling of Primary Allylic Amines with Boronic Acids and Boronates through Palladium‐Catalyzed CN Bond Cleavage - Li - 2012 - Angewandte Chemie International Edition - Wiley Online Library
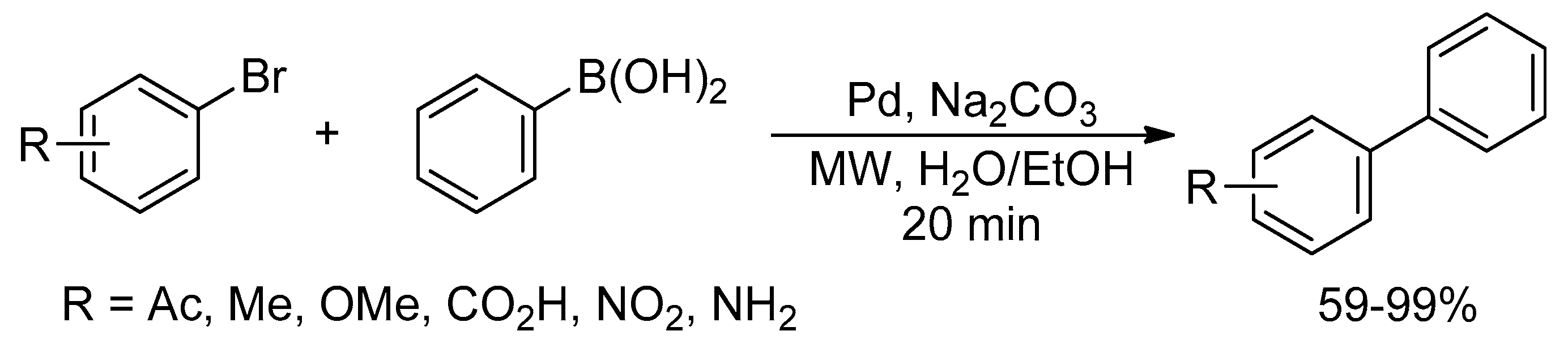
Catalysts | Free Full-Text | Microwave-Assisted Palladium-Catalyzed Cross-Coupling Reactions: Generation of Carbon–Carbon Bond | HTML

EP2107047A1 - Method for producing organic compounds by means of a transition metal-catalysed cross-coupling reaction of an aryl-X, heteroaryl-X, cycloalkenyl-X or alkenyl-X compound with an alkyl, alkenyl, cycloalkyl or cycloalkenyl halogenide -

Palladium(II)-catalyzed C-H activation/C-C cross-coupling reactions: versatility and practicality. - Abstract - Europe PMC
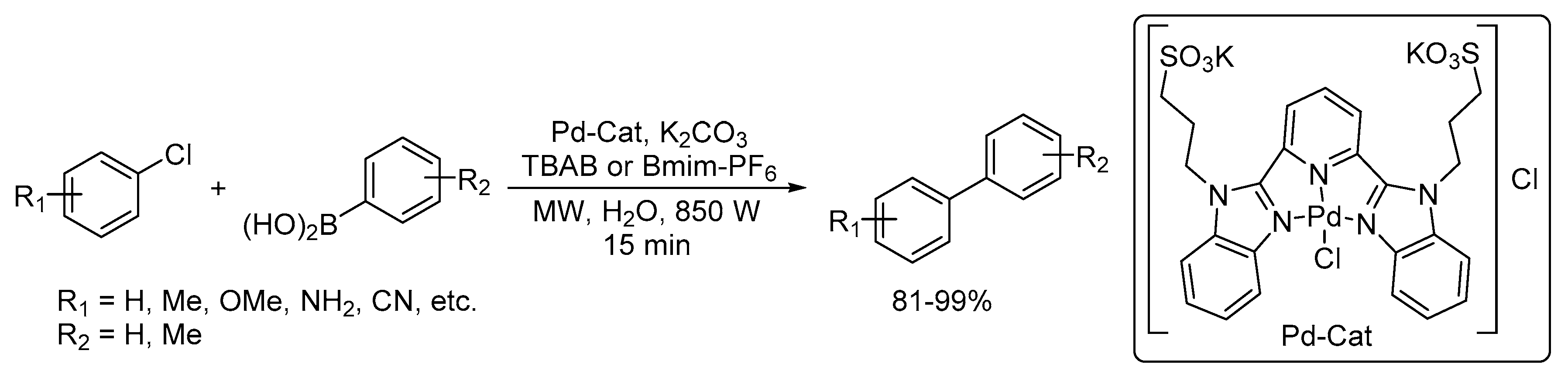
Catalysts | Free Full-Text | Microwave-Assisted Palladium-Catalyzed Cross-Coupling Reactions: Generation of Carbon–Carbon Bond | HTML

Palladium-catalyzed asymmetric dearomative alkenylation of indoles through a reductive-Heck reaction - Organic Chemistry Frontiers (RSC Publishing)
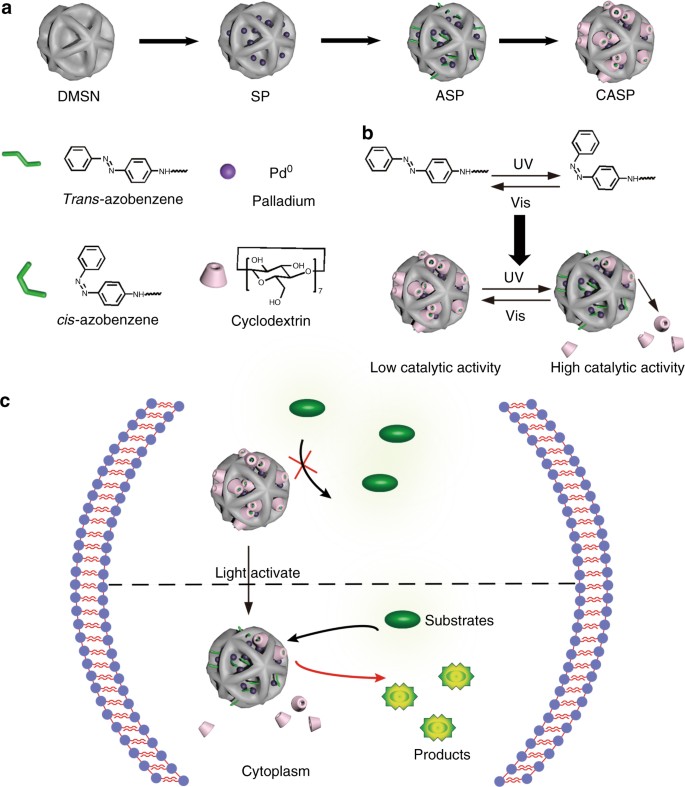
Designed heterogeneous palladium catalysts for reversible light-controlled bioorthogonal catalysis in living cells | Nature Communications